Outrigger Kona Resort and Spa in Kona, Hawai’i September 22 – 24.
Dr Jason Lowe presented his paper in a brief 15-minute talk.
Histologic and cytologic changes in normal equine joints after injection with 2.5% injectable polyacrylamide hydrogel (2.5 iPAAG).
Jason Lowe1*, Leigh de Clifford2, Alan Julian3, Janet Patterson-Kane4, Marc Koene5.
1IMS Vet, Cambridge, New Zealand.
2Matamata Vet Services Equine, Matamata, New Zealand
3Idexx Laboratories, Hamilton, New Zealand
4Animal Strategies LLC, Denver, CO, United States.
5Tierklinik Lusche Veterinary Hospital, Bakum, Germany
Osteoarthritis (OA) is a leading cause of joint pain and lameness in humans and animals and has significant economic and welfare considerations as a result. Multiple clinical and experimental studies have demonstrated that intra-articular 2.5% Polyacrylamide Hydrogel (2.5 iPAAG- Arthramid Vet®) is long-lasting (up to 24 months), safe, and highly effective at alleviating the clinical signs of OA. The gel is integrated into the synovial membrane but is chemically inert supporting the notion that it exerts its effects on joints through purely mechanical mechanisms.
In this study, 10 healthy horses not suffering from OA or signs of joint disease were administered 50 mg or 100 mg 2.5 iPAAG in a total of 13 joints. Joints were examined 0, 14, 42, and/or 90 days post-injection. Parameters investigated included synoviocentesis, gross pathology, histology, and scanning electron microscopy.
All horses remained clinically normal with no adverse events recorded throughout the study period and gross post-mortem did not reveal any significant findings. Moreover, arthrocentesis cytology parameters remained within clinically normal levels throughout the study.
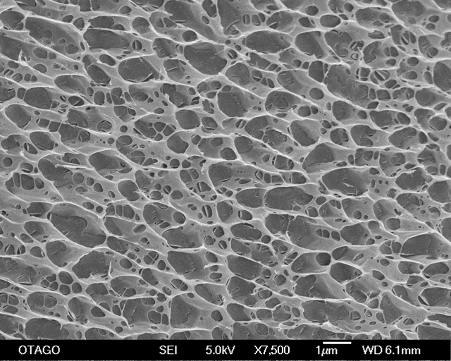
Synovial histology demonstrated that cellular infiltration, hyperplasia, and vascularisation were significantly higher in 2.5 iPAAG treated joints compared to controls and scanning electron microscopy confirmed that the 2.5 iPAAG demonstrated an extensive tissue integration as a 3-dimensional scaffolding structure with intact cross-linked strands.
These results confirm that an intra-articular injection of the 2.5 iPAAG induces a typical foreign body response which is predominately macrophage driven, and with no evidence of fibrosis, granuloma or mineralization. These findings are clinically relevant in helping our understanding of how to effectively manage horses after treatment, or in a rare case (< 1:2500) of non-septic flare reaction.
Furthermore, these observations were discussed in light of recent advances in cell-omics that have identified a rich heterogeneity in synovial tissue macrophage (STM) populations. Unique clusters of STM’s act as a protective lining barrier within the synovial membrane in humans with rheumatoid arthritis. The idea of a treatment such as 2.5 iPAAG that acts as a bio-scaffold and through its presence mechanically stimulates endogenous resolution of inflammation by increasing the innate homeostatic mechanisms of the joint through the recruitment of pro-resolving macrophages, rather than simply blocking inflammation through the use of non-steroidal anti-inflammatory drugs or corticosteroids, could represent a paradigm shift in veterinarians thinking about the treatment of OA.